How do I submit an inquiry or service request?
Please use the Contact Us form under the About Us tab, where you can provide your contact information and details about your project.
Please include which target protein(s) you wish to study, the quantity of compounds you wish to test, the type of assay you wish to use (if known), and any other important details of your scope of work.
Alternatively:
Contact Customerservice@lifesensors.com for product and technical inquiries.
Contact bd@lifesensors.com for CRO services, custom product/service requests, and other custom inquiries.
Does LifeSensors have international distributors?
Yes, LifeSensors has many international distributor partners. International customers (non-US) should contact their local distributor for product orders. For a list of LifeSensors distributors, visit our Distributor Page.
Where can I access details on the protocol for the reagent or assay kit I ordered?
Details on the protocol for the product or assay kit can be found on each product page under ‘Documents’. Additional information and technical help are available by contacting customerservice@lifesensors.com.
Where can I access details on the protocol for the reagent or assay kit I ordered?
Product datasheets can be found on each product page under the ‘Documents’ tab.
How do I freeze-thaw TUBEs and recombinant proteins?
TUBEs and recombinant proteins should be limited to minimal freeze-thaw cycles. Once received, the product should be aliquoted appropriately based on usage and stored in the appropriate temperature as stated on the website.
How do I convert mg/mL mass concentration to Molar concentration?
To convert mg/mL to molar concentration, you will first need to acquire the molecular weight of the protein. You then divide the concentration by the molecular weight.
For example, wild type ubiquitin has a molecular weight of 8.56 kDa. Molar concentration of a 5.0 mg/ml solution of wild type ubiquitin can be calculated as follows; 5.0/8.56= 0.584 mM or 584.0 µM.
What are storage conditions for recombinant proteins?
Recombinant proteins should be stored at -80°C.
How do I send samples for screening?
We can receive compounds in powder form, as DMSO stocks or as aqueous solutions. Compounds can be shipped to us as individual vials or in 96-well or 384-well plates.
Sample shipment address:
LifeSensors, Inc.
ATTN: Karteek Kadimisetty
271 Great Valley Parkway
Malvern, PA 19355
Can I get a custom protein tag or a custom labelling for my E3 or DUB of interest?
Yes. We can provide E3 or DUB with custom protein tag or custom labeling. Please contact bd@lifesensors.com for custom product/service requests.
How do I approach using SUMO-tag for commercial applications with a license?
Please contact bd@lifesensors.com for licensing inquiries.
PROTAC
What is a PROTAC?
PROTAC stands for Proteolytic Targeting Chimera that has multiple functional units that engage specific proteins of interest within the cells so that can be degraded using cell’s own ubiquitin proteasome system. One of the ligand pf the PROTAC typically refers to “warhead” targets protein of interest and another ligand often refers to “anchor” is used to recruit E3 ubiquitin ligase. Both the warhead and anchor are connected by a chemical linker making a PROTAC.
What are different types of assays LifeSensors offers for PROTAC Drug Discovery?
LifeSensors offer a suite of assays that characterizes a PROTAC from its affinity to bind with proteins of interest to cellular assays that can demonstrate degradation and selectivity.
1. Biophysical assays to monitor ternary complex formation and validating ligand affinity
2. In vitro ubiquitination assays to validate a functional ternary complex
3. Cell based ubiquitination assays to validate cell permeability and function in cells
4. Degradation assays to validate degradation and demonstrate DC50 & selectivity
5. Mass spectrometry to demonstrate selectivity
Do you offer PROTAC design and Medicinal Chemistry?
Yes, LifeSensors offer medicinal chemistry services to design and synthesize PROTACs with novel ligands and demonstrate ubiquitination and degradation in complementary assays.
Does LifeSensors offer services to screen ligands for novel E3 ligases for PROTAC applications?
Yes, LifeSensors has access to 35 E3 ligases where clients can perform rapid screening to identify novel ligands for PROTAC applications. LifeSensors is equipped with state-of-the-art robotics that offers faster turnaround from identifying hits to further validate them in an array of assays.
Does linker length and linker type are crucial for effective PROTAC design?
PROTAC linker is one of the most underestimated factors when a PROTAC is being designed. Both Linker length and linker composition do play crucial role in getting optimal PROTAC efficiency. Multiple studies have highlighted new generation of functional linkers explored with more complexity are offering ternary complex stabilization subsequently improving degradation.
What is the principle of LifeSensors' PA950 PROTAC Assay Plate?
The PROTAC® Assay Plate is a sandwich-based assay in which polyubiquitinated proteins from cell lysates are captured in the wells of a precoated microtiter plate using a proprietary polyubiquitin binding reagent. Proteins that are not polyubiquitinated/unbound are removed by washing and then an antibody directed against the target protein is added followed by washing. Lastly, a secondary antibody conjugated to horse radish peroxidase (HRP) is used to measure the bound target antibody with detection reagents and a luminescence microplate reader.
Learn more here.
What is the principle of LifeSensors' PA770 In Vitro Ubiquitination Assay Kit?
The In vitro ubiquitination kit has been developed to establish a high throughput approach that can accurately predict PROTAC efficiency by monitoring the protein’s intrinsic ability to get ubiquitinated. We offer this kit for three E3 ubiquitin ligases: Cereblon, VHL, and HDM2 to monitor PROTAC mediated ubiquitination for target of choice. At the core of the assay, microtiter plate strips, pre-coated with a proprietary TUBE reagent (assay plate) are used for the capture of polyubiquitin chains formed in a PROTAC dependent reaction.
The signal generated by captured polyubiquitylated product in this “sandwich” ELISA-like assay is a quantitative measure of PROTAC activity. Furthermore, this detection strategy does not require additional non-native tagging or labeling of ubiquitin, which could lead to experimental artifacts.
Learn more here.
What type of polyubiquitination can be detected with LifeSensors' PROTAC Assay Plate?
Since our plates use pan-selective TUBEs, any type of polyubiquitination with any of seven lysine residues (K6, K11, K27, K29, K33, K48, K63) will be detected.
Does LifeSensors' PROTAC Assay Plate work for Molecular Glue discovery?
Yes, our PROTAC Assay Plates, along with our In vitro ubiquitination assay kit, have been designed to work with both PROTACs and Molecular Glues.
What is the PA950 Decomplexing Buffer?
For some targets, the decomplexing agent enhances the signal to background. We recommend the use of the decomplexing agent prior to analysis with PA950. The use of the decomplexing agent disrupts any native protein complexes that might be part of ubiquitin complexes. Using this urea based decomplexing agent results in reduced background signal, resulting in a better signal-to-background ratio. This buffer is available for purchase on the LifeSensors website here.
TUBEs
What are the different types of pan-TUBEs LifeSensors sells?
LifeSensors’ TUBE (Tandem Ubiquitin Binding Entity) technologies are industry-leading tools to detect and enrich/purify poly-ubiquitylated proteins from cell lines, tissues, and organs. LifeSensors has two commercially available pan-TUBEs (TUBE1 and TUBE2). Both TUBE1 and TUBE2 come in various flavors such that they can be used for far-Western detection (FLAG, Biotin), enrichment/purification (linked to Agarose and Magnetic beads), and in vivo imaging (TAMRA, FITC-attached) applications. For more information, please see our TUBEs page.
What is the difference between TUBE1 and TUBE2?
TUBE1 and TUBE2 are made from different ubiquitin-binding domains (UBDs). However, both of them have been shown to bind all the linkages of polyUb with single digit nM Kd. TUBE1 has been shown to preferentially bind K63-polyUb over K48-polyUb. TUBE2 binds both K48- and K63-polyUb with equal affinities.
What is the difference between K48 TUBE and K48 TUBE HF?
LifeSensors have recently developed a new K48 TUBE HF (high-fidelity) that has comparable affinity (Kd of ~20 nM for tetra-ubiquitin) to their cognate poly-Ub as our existing K63 TUBE and M1 TUBE. The old K48TUBE binds tetra-ubiquitin with a Kd of ~200 nM.
Which TUBE is more efficient at capturing human proteins as opposed to rodents, plants, or yeasts?
TUBEs bind to ubiquitin/polyubiquitin chains which are highly conserved across all eukaryotes. However, the poly-ubiquitin linkage type (K48, K63) and the relative abundance of target protein should be considered while making a selection of TUBEs for your experiments.
Have TUBEs been tested for plants and other organisms?
No, TUBEs have not been tested by LifeSensors for organisms other than mammalian and yeast ubiquitin/poly-ubiquitins. Since ubiquitin is highly conserved from yeast to human, theoretically TUBEs should work for plants and other organisms. We welcome any feedback from customers who have studied other organisms with our TUBEs.
How much protein (cell/tissue extracts) do I need to add to TUBEs?
The abundance of the target protein and the type of sample (cells, tissues, organs) should be considered to estimate the amount of cell/tissue extracts needed. As a starting point, we recommend to use 20 µL of agarose -TUBE beads or 100 µL magnetic-TUBE slurry per milligram of cell extract. The amount of cell/tissue extracts, volume of the eluate, and the volume of the agarose/magnetic beads should be optimized by the end user.
How do I elute poly-ubiquitylated proteins from TUBEs?
Elute poly-ubiquitylated proteins from agarose/magnetic-TUBEs using our proprietary elution buffer (LifeSensors Cat # UM411B). Alternatively, for gel-based separation and subsequent applications, the ubiquitylated proteins can be eluted using a standard loading dye for SDS-PAGE.
How do I elute poly-ubiquitylated proteins for mass spectrometric analysis?
Buffers containing chaotropic agents, such as urea and guanidinium-HCl, can be used for eluting poly-ubiquitylated proteins for subsequent mass spectrometric analysis. Alternatively, poly-ubiquitylated proteins can be eluted using SDS-PAGE loading buffer, separated by SDS-PAGE and subjected to trypsin digestion prior to MS analysis.
What are the controls for TUBEs function?
Positive control: How do I know that TUBEs work?
The properties of TUBEs have been well characterized and published. Each TUBE lot is quality controlled for overall binding capacity and the pull-down efficiency of polyubiquitylated proteins using cell lysate.
Negative control: How do I know that TUBEs aren’t yielding an artifact?
We recommend to include a negative control in your pull-downs that contains the quenched agarose resin (LifeSensors cat # UM400).
Can I enrich mono-ubiquitylated proteins with TUBEs?
It is possible that under certain conditions significant amounts of mono-ubiquitylated proteins can be enriched/purified using TUBEs. This is particularly true for more abundant mono-ubiquitylated proteins. However, under typical cellular conditions, the majority of the isolated ubiquitins are likely to be polyubiquitylated.
Does LifeSensors have a TUBE product to detect SUMOylated proteins?
Yes, we do have SUMO capture reagent (LifeSensors Cat # SM101) to detect and enrich/purify SUMOylated proteins from cells, tissues, and organs.
What are the assays developed by LifeSensors using TUBE technology?
LifeSensors has successfully applied the TUBE technology to detect and quantify the ubiquitylation status of a tagged/endogenous protein (UbiTest, UbiQuant S), detect the ubiquitin linkages (linkage-selective UbiTest), and quantify the ubiquitylated target protein in a high throughput format (HT-UbiTest).
What is UbiTest?
The UbiTest platform replaces traditional immunoblotting methods for measuring cellular ubiquitylation, enabling simple comparisons of substrate ubiquitylation levels between samples and determination of ubiquitin chain linkage types. UbiTest is based on TUBE technology for enriching polyubiquitylated proteins. By visualizing the ubiquitylated fraction both with and without DUB digestion of polyubiquitin chains, UbiTest streamlines the confirmation of ubiquitylation status. For more information about UbiTest, please also visit our UbiTest product pages, where you can purchase off-the-shelf assay kits for performing UbiTest in your own lab.
LifeSensors has also developed High-Throughput (HT) UbiTest which is a customized medium-throughput assay for absolute quantification of ubiquitylated substrate levels in cells that combines UbiTest principles and TR-FRET technology. Please contact us if you like to develop similar assays for your target protein.
What is UbiQuant S?
LifeSensors’ UbiQuant S ELISA and AlphaLISA assays are industry-leading platforms for measuring ubiquitylation of a target protein in cells. These assays utilized TUBEs (Tandem Ubiquitin Binding Entities) to detect ubiquitylated substrates, and are individually optimized for a specific substrate protein.
Does LifeSensors offer services to develop a customized assay for a target protein?
Yes, LifeSensors offers services to customize and develop assays required for your target protein. Additionally, LifeSensors has the expertise to determine compound activity in a cellular context. Our assays for monitoring cellular ubiquitylation will help you quickly demonstrate the downstream effects of your E3 ligase or DUB inhibitor. Ranging in throughput from immunoblot to 384-well formats, our assay concepts can be readily adapted for a vast range of substrates on a custom basis. Thus, our technologies will enable the advancement of your compounds from in vitro lead compounds to in vivo and preclinical models. For more information, please contact us or visit our service pages.
Does LifeSensors offer TUBE-based proteomics services?
Yes, LifeSensors offers TUBE-based proteomics services. LifeSensors have developed two proprietary technologies to help researchers accomplish their scientific goal – (1) identify cellular proteins that are ubiquitinated and characterize the ubiquitin-linkages on them (TUBE proteomics), and (2) qualitative and quantitative profiling of ubiquitination sites on proteins (UbiSight proteomics). For more information, please contact us or visit our service pages.
What are the storage conditions for Magnetic Beads?
Magnetic beads should be stored at 4°C after arrival. After use any unused beads should be stored with PBS + 20% EtOH.
What are the storage conditions for Agarose Beads?
Agarose beads should be stored at 4°C after arrival. Do not freeze the agarose beads.
Are there loading controls for Western blots after TUBE enrichment?
Unlike with whole cell lysate, probing for a housekeeping protein, like GAPDH, is not possible after TUBE enrichment. Thus, we recommend completing pulldowns from all experimental conditions using the same quantity of lysate and the same amount of TUBE.
When should I use a solution-based pulldown vs. a matrix-based pulldown?
Using a matrix in TUBE pulldowns provides a solid support for specific interactions, enabling high specificity and efficient ubiquitinated protein enrichment. Solution pull-down methods offer flexibility in studying protein interactions in a more native environment. This approach allows for the investigation of dynamic interactions between proteins without the constraints of being bound to a solid support. It’s particularly useful when studying transient or weak interactions that might be disrupted in the rigid conditions of a solid-phase matrix.
What are the differences between Agarose TUBEs and Magnetic TUBEs?
Magnetic TUBEs offer various advantages over agarose TUBEs. Agarose TUBEs require centrifugation steps for separation of beads from the solution phase. Magnetic beads offer an advantage in terms of ease of separation, as well as their ability to be used in bead-based ELISAs.
What is the difference between Magnetic TUBE1 and High-Capacity Magnetic TUBE1?
High-Capacity TUBE1 (UM501M) were designed by coating polymeric high-capacity magnetic beads to allow superior enrichment of polyubiquitinated proteins along with minimizing non-specific binding to proteins in tissue and cellular lysates. Magnetic TUBE1 beads (UM401M) are 1 µm and High-Capacity TUBE1 beads (UM501M) are 2.8 µm in diameter.
How do I perform a TUBE pulldown?
Depending on the type of TUBE purchased, the protocol differs slightly. Please refer to the manual for each specific product when performing a pulldown. This manual can be found on the product page for each specific TUBE.
For Western blotting, what antibodies should I use?
For western blotting, to detect overall ubiquitination signal, use VU-1 antibody. To detect target ubiquitination, use a target specific antibody.
How do I reduce background and non-specific binding in TUBE pulldowns?
To reduce background and non-specific binding, we recommend preincubating the TUBEs in 3% BSA for one hour at 4 °C followed by three washes with PBST.
Can I use TUBEs as detection reagents?
Yes. TUBEs can be used as detection reagents in both Western blotting and ELISA-based assays. LifeSensors offers an HRP-conjugated TUBE2 for easy, one-step detection of pan-ubiquitination. Additionally, we offer biotin-labeled TUBEs (M1 Biotin TUBE, TUBE1 Biotin, TUBE2 Biotin, K63 Biotin TUBE, and K48 HF Biotin TUBE) for detection with streptavidin-HRP.
SUMO
What is the advantage of using the SUMO system?
Many engineered/designer proteins or single-chain antibodies are poorly expressed. Fusion with SUMO/SUMOstar enhances the stability, solubility, and yield of a protein. SUMO is one of the most conserved proteins in eukaryotes and have been shown to post-translationally modify a number of proteins in cells. SUMOylation plays an important role in various cellular process such as nuclear-cytosolic transport, transcriptional regulation, and protein stability. SUMO/SUMOstar-fusion leads to a dramatic enhancement of expression in E. coli, yeast, insect, and mammalian cells and promotes correct folding and hence biological activity. Additionally, this system simplifies and lowers the costs associated with protein expression and purification. Using this technology, a plethora of proteins have been shown to enhance their expression, stability, and yield.
What proteins have been previously expressed with SUMO?
SUMO has been used with a range of proteins for a variety of purposes. We included a table attached to our SUMO technology page that has a range of proteins, as well as additional information from each scientific publication. SUMO system has been used at more than a thousand labs around the world. Almost all members of each protein family have been expressed using the SUMO system in a variety of hosts. We encourage reaching out, as we have included a partial list of references, but our library is growing constantly in this cutting edge of research.
What is the difference between SUMOpro and SUMOstar?
SUMOstar extends the benefits of our SUMO system to eukaryotes. Therefore SUMOstar is preferred expression system for all difficult to express proteins in mammalian and other eukaryotic cells. SUMOstar fusion cannot be cleaved with SUMOprotease or SUMO protease II as the tag is engineered not to be cleaved. The SUMOstar tag can only be cleaved with SUMOstar protease.
What host models are available?
We offer systems that are optimized for:
– Bacterial: E.coli
– Yeast: S.cereviseaand P.pichia
– Baculoviral/Insect cell system
– Mammalian: HEK293and CHO cells
What is the SUMOpro3 system?
LifeSensors discovered that human SUMO is superior for E.coli expression of some of the proteins in E.coli. Therefore we developed human SUMO system and called it SUMOpro3. SUMOpro3 tag is best cleaved with SUMO protease 2, that is a human de-SUMOylase. Please contact us to help you evaluate the best system for your specific needs.
LifeSensors discovered that human SUMO is superior for E.coli expression of some of the proteins in E.coli. Therefore we developed human SUMO system and called it SUMOpro3. SUMOpro3 tag is best cleaved with SUMO protease 2, that is a human de-SUMOylase. Please contact us to help you evaluate the best system for your specific needs.
What is included in a SUMO kit?
Our SUMO system kit offers everything you need to express your protein of interest in a given host. The kits include a SUMO plasmid where you can insert your gene of interest, the SUMO protease to cleave the SUMO tag, the SUMO protease control protein, and an antibody to detect our SUMO tag. The kits include a manual featuring a polylinker map of the vector for an easy cloning process.
What is the best way to ensure the protease removed the SUMO tag?
LifeSensors offers several antibodies to detect the SUMO tag. This allows you to check the efficiency of the protease cleavage by simple western blot methods. We recommend using this as a quality checkpoint when optimizing your expression system and protein purification. We are happy to help you implement best practices for protein expression and provide recommendation to get a high yield of active
What is the SUMO tag system?
Attachment of the C-terminus of SUMO (Small Ubiquitin Like Modifier) to the N-terminus of a protein of interest can dramatically improve protein solubility, achieve native protein folding, and increase total yield by improving expression and decreasing degradation. LifeSensors protein expression system has pioneered SUMO fusion tag technology, which increases the quantity and quality of numerous recombinant proteins in both prokaryotic and eukaryotic expression hosts. Our cleavable SUMO tag system allows production of recombinant proteins with their native N-terminus intact and without any unwanted residues. We offer a protein expression service related to the expression and purification of proteins.
How do I approach using SUMO-tag for commercial applications with a license?
Please contact bd@lifesensors.com for licensing inquiries.
Kits and Plates
What lysis buffer should I use for cell lysis?
For cell lysis, use RIPA buffer with 1 mM PMSF, 20 µM MG132, 50 µM PR619, 5 mM 1,10 phenanthroline, Protease inhibitor cocktail 1 to 500 dilution (Sigma Cat#P8849).
Can I use LifeSensors' plates only for PROTACs?
No! LifeSensors’ plates work for bivalent and trivalent PROTACs as well as molecular glues.
What E3 ligases are offered for PA770 In vitro ubiquitination assay kit?
The E3 ligases offered for PA770 In vitro ubiquitination assay are Cereblon, VHL, and HDM2. Custom Kits can be created for other E3 Ligases as a separate offering.
What secondary antibodies are offered for PA770 In vitro ubiquitination assay kit, and how do I know which one to use?
We offer mouse and rabbit secondary antibodies. Which one you use will depend on your primary antibody and in which organism it was raised.
DUBs and Ligases
What is a DUBTAC?
DUBTACs, or DeUBiquitinating TArgeting Chimeras, recruit DUBs to a target protein and remove ubiquitin chains, resulting in stabilization of target proteins.
DUBTACs consist of three components:
- DUB recruiter
- Target protein binder
- Linker connecting both entities
DUBTACs restore protein levels, function, and rescue target proteins from degradation via the proteasome.
Do you offer custom assay development for DUBs and E3 ligases?
Yes. Lifesensors has several years of experience in developing and validating custom assays for DUBs and E3 ligases. The assays can be used in high throughput screening to discover small molecule modulators.
What is the ideal substrate for measuring DUB activity?
What is the ideal substrate for measuring DUB activity?
The majority of the DUBs show activity using the Ub-Rh110 substrate. However, some DUBs prefer Di-Ubiquitin substrates. LifeSensors offers K6-DiUb, K11-DiUb, K48-DiUb and K63-DiUb TR-FRET substrates for testing DUB activity as well as chain selectivity of DUBs.
How do I choose a DUB panel for testing selectivity of my compounds?
Lifesensors offers two separate panels of DUBs for testing compound selectivity. DUB Panel I comprises of 10 DUBs and DUB Panel II contains 22 DUBs. DUB Panel I is ideal for initial testing of compounds. Testing can be done in a single concentration format or dose response format to obtain IC50s.
How do you measure E3 ligase activity?
Lifesensors utilizes its TUBE-based homogeneous TR-FRET assay to measure E3 ligase activity. Biotin-TUBEs bind to the fluorescently labeled polyubiquitin chains synthesized by the E3 ligase resulting in a TR-FRET signal. This assay can measure E3 auto-ubiquitination or substrate ubiquitination.
How do I choose an E3 ligase panel for testing selectivity of my compounds?
Lifesensors offers three separate panels of E3s for testing compound selectivity. There are 5 E3s in Panel I, 10 E3s in Panel II and 30 E3s in Panel III. Initial selectivity testing can be done using Panel I which comprises of members from different classes of E3s. Testing can be done in a single concentration format or dose response format to obtain IC50s.
How do you prefer to receive compounds for testing?
We can receive compounds in powder form, as DMSO stocks or as aqueous solutions. Compounds can be shipped to us as individual vials or in 96-well or 384-well plates.
Do you sell inhibitors or agonists to use as an internal control in biochemical assays?
We sell inhibitors for use as internal controls for several DUBs.
Why doesn't my assay work?
The DUB and E3 assays require reducing agent for maintaining their catalytic activity. We recommend adding 2mM beta-mercaptoethanol or 10mM DTT to the assay buffer and using this assay buffer to make enzyme and substrate dilutions as well as performing the assays. E3 assays contain multiple components and care must be taken to ensure that all the necessary components like E1, E2, E3, Ubiquitin and ATP are included in the assay.
Ubiquitin & Ubiquitin Chains
What assay buffer do I use for testing my chains with DUBs?
We recommend 50 mM HEPES, 100 mM NaCl, and 5 mM DTT, pH = 8.0. DTT ensures that the active site cysteine of the DUB is reduced, allowing DUBs to perform their best. When screening compounds in the assay, the addition of detergents such as CHAPS at 0.05% and Bovine Serum Albumin (BSA) at 0.01% can improve the assay performance and the quality of hits. Assay buffers containing 50mM Bicine or 50 mM Tris, pH = 8.0, can also be used. 1-2mM β-mercaptoethanol can also be used as reducing agent instead of DTT. Although these recommended assay buffers should yield the best results for cleavage of polyubiquitin chains with most DUBs, certain DUBs may require additional buffer optimization and/or cofactors/binding partners for best performance.
What temperature should I store my chains at?
We keep all of our chains at -80°C here at Lifesensors, but they are safe to thaw on ice. Do not leave them out for extended periods of time and always return them to -80°C when finished for the day.
Why do I see multiple bands on my gel/Western Blot while cleaving?
Depending on the activity of the DUB and which DUBs you are using, you can get complete or partial cleavage. Always include a “-DUB” control to see what the chain looks like before cleavage. If you cleave a tetra-ubiquitin, for example, then you should end up with a gel that has a band at Tetra-, Tri-, Di-, and monoubiquitin. This means the DUB successfully cleaved the ubiquitin. If you see only monoubiquitin and you started with a tetra-ubiquitin, then you had 100% cleavage.
If you are just running a Western blot, we do not recommend loading more than 50 ng at a time as overloading could yield “smudged” Western Blot results.
Will my chains get damaged after multiple freeze thaws?
No, freeze thaws should not affect any chain ubiquitin. Freeze thaws will, however, affect the DUBs used to cleave them as they are enzymes. The DUBs will begin to lose activity after multiple freeze thaws. If you are worried about your chains or your DUBs, aliquot your desired amounts and thaw as needed.
If I purchase the "Non-Cleavable" Ubiquitin and a "DUB-Resistant" Ubiquitin, what should I expect, and what is the difference between the two?
Non-Cleavable DUB-Resistant Ubiquitin Chains are linear, recombinant proteins expressed and purified from E.Coli. They are modified to be resistant to even the most promiscuous DUBs. When incubated with a high DUB concentration, normal chains show almost 100% cleavage, while DUB-Resistant Chains exhibit little to no cleavage at 100 nM DUB concentration.
What is the difference between Di, Tri, and Tetra chains and the others?
Linear ubiquitin chains are recombinantly expressed and purified from cells. Linear chains do not have specific linkages unlike non-linear chains. Any K6, K11, K27, K29, K33, K48, and K63 linked Di, Tri, and Tetra Ub chains are made enzymatically in vitro. We use a combination of chain specific E2s and DUBs to first assemble the chains and then remove unwanted chains before purifying the specific chains.
What are K to R Ubiquitin Mutants listed on your website?
K to R mutants are ubiquitin mutants that have their individual lysines at K6, K11, K27, K29, K33, K48, K63, or all (K0) lysines mutated to arginine (R). This is to generate different types of ubiquitin chains depending on the end user’s goals.
Why are mutations/linkages at lysines?
Ubiquitin comprises 7 lysine residues among its 76 amino acids. The attachment of ubiquitin to specific lysine residues in proteins, including itself, is crucial for creating diverse substrate-ubiquitin structures. This versatility allows the pathway to target proteins for various fates, such as degradation or control of specific cell cycle phases. For instance, K48 chains promotes proteasomal degradation, K11 regulates substrates in the anaphase-promoting complex, and K63-linked ubiquitination controls the interaction, translocation, and activation of proteins.
What is the purpose of products like UbRh110, UbAMC, K48-UbRh110 etc.?
Ubiquitin Rhodamine and AMC are ubiquitin derivatives used as substrates to test the activity of DUBs. When the DUB cleaves Ubiquitin from Ub-Rhodamine or UbAMC, the release of free Rhodamine and AMC results in an increase in fluorescence. These substrates allow for simple, fast, quantitative, and real-time monitoring of DUB activity.
K48 Tetra Ub linked Rhodamine is the same as above but meant for testing DUBs that are specific to K48 chains.
Mass Spectrometry
What are the advantages of TUBE-based mass spec?
Tandem Ubiquitin Binding Entities (TUBEs) are industry-leading tools to detect and enrich/purify poly-ubiquitylated proteins from cell lines, tissues, and organs. Pairing this sample processing step with LC/MS analysis, generates a reproducible survey of the ubiquitome. We have worked with clients to investigate ubiquitin levels as a response to drug dosing, genetic knockouts, disease states, and others.
What are the disadvantages of traditional methods?
Traditional Mass spec proteomics methods do not work well for the ubiquitin proteome system. Ubiquitylation of proteins is very dynamic and sample preparation to preserve pattern of ubiquitylation is critical. Above all, methods used to enrich ubiquitylated peptides by di-Gly-Lys antibody fail to capture all the ubiquitylated proteins. Because of this, traditional approaches have proven to be irreproducible.
What can I expect to get out of a mass spec service?
Clients receive their processed data in an excel file, as well as a summary report. We include graphical representations of ubiquitylation tends of your proteins. You will receive professional support when analyzing your data. See example report.
- Written report with summary of the TUBE based mass spec proteomics data, with sample prep protocols and LC-MS methods implemented for given samples along with sample description.
- Total Ubiquitinated identified will be presented in a list, as well as being organized on a distribution chart to show proportions.
- Total peptides identified will be presented in a list with length and mass information.
- Additional bioinformatic analysis for getting functional perspective of Proteomics data like DE analysis, cluster analysis and pathway & network analysis are available upon request at addition costs.
What is the typical workflow of LifeSensors' mass spec service?
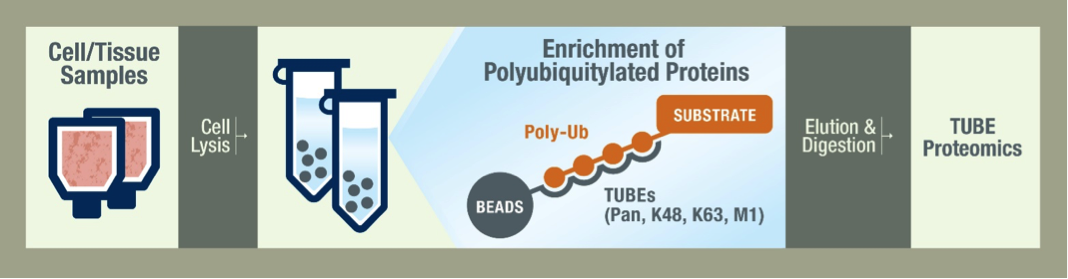
Your job ends at the shipment of frozen cells or tissue. Our scientists lyse your cells with a cocktail of inhibitors to protect the ubiquitylated species. The samples are then prepared using the TUBEs to pulldown the ubiquitylated proteins. Pan selective TUBEs are recommended for a global view of the ubiquitiome, but we also offer K48 and K63 linkage-specific TUBEs for specialized studies. Proteins are then separated on a gel by SDS-PAGE and are stained for analysis. After data is collected by LC-MS/MS we begin data analysis by running bioinformatic searches. The depth of our analysis depends on your needs and budget and we are happy to work with you to decide the best methods.
What is the required amount of starting material?
We require sample of 1mg-5mg of total protein for this analysis. Depending on your cell type this will equate to pellets of approximately 50 million cells. If you are using tissue samples, we can discuss sample sizes that would be sufficient.
Do you recommend duplicates? Triplicates?
We recommend submitting at least two samples for each condition for a more robust analysis. If you are looking to publish this data, duplicates are typically enough for this type of experiment. Ensure that you design your experiment with the proper controls, and in duplicate in order to have enough data to draw meaningful conclusions.
What is the turnaround time?
We are typically able to deliver a report after 2-3 weeks upon receipt of the samples. Depending on the number of projects at any given time this is subject to change. Please contact us to get a more accurate turnaround time for your specific project.
What type of control samples do you recommend?
The controls for each experiment depend on the question being asked, but there are some guidelines that you might find useful. If you are looking into the effects of a drug or other compound, we recommend submitting a negative control. For genetic studies the negative control may be a wildtype sample. The use of inhibitors is another important control in this type of study. We recommend submitting samples treated with proteasome inhibitors, such as MG-132 to accumulate ubiquitylated proteins. We are happy to help you design a robust experiment around your specific research question, please contact us.
Is this analysis quantitative?
Yes, the TUBE-based mass spec analysis is quantitative. The intensities reported describe the abundance of that peptide or protein. These values account for a loading control, as well as the size of the proteins.
Thermal Shift & SPR Assays
What is thermal shift assay (TSA)?
A protein thermal shift assay, sometimes called a thermoflour assay, measures changes in the thermal denaturation temperature (ΔTm, °C) of a protein resulting from the binding of small molecules. The assay is rapid and can be used for high throughput screening to identify and characterize small molecule compounds that bind to proteins.
What is the principle of thermal shift assay?
When a protein is gradually heated, the melting/denaturation of the protein exposes hydrophobic regions. In the presence of a dye (e.g. SYPRO Orange) the hydrophobic regions will bind to the dye, resulting in a pronounced increase in its fluorescence intensity. The temperature at which a protein is 50% denatured is called the melting temperature (Tm) of the protein. Binding of small molecules can either increase or decrease the Tm of the protein; we are measuring the change in the melting temperature relative to untreated protein.
How much protein is needed in a thermal shift assay?
Typically, 1-5µM of target protein is sufficient to generate strong signal in the thermal shift assay. However, the optimal concentration for individual proteins should be experimentally determined prior to any screening or compound testing.
What equipment is needed for running a thermal shift assay?
A thermal cycler that can hold 384-well plates and measure fluorescence is needed for thermal shift assay based high-throughput screening.
Do thermal shift assays work on all proteins?
While thermal shift assay should work with most proteins, some proteins may not be suitable for thermal shift assay. Smaller proteins with very low hydrophobicity or proteins that are not folded/aggregated are not suitable for thermal shift.
What solvent is used for thermal shift dye?
SYPRO Orange is dissolved in DMSO.
What is SPR Assay?
Surface Plasmon Resonance (SPR) is an optical technique for detecting the interaction of two different molecules. One molecule (the ligand) is immobilized on a gold-coated surface, while another molecule (the analyte) is injected over the surface. The technique measures a change in refractive index at the surface, which is directly proportional to the change in mass. Both protein-protein and protein/small molecule interactions can be investigated with this method. Binding response units (RUs) are measured in real-time and analyzed to determine kinetic parameters including the binding affinity (KD).
What are the different types of protein capturing methods you use in SPR?
We routinely use amine-coupling to immobilize proteins on to SPR sensor chips. We also use high affinity nickel chips to immobilize proteins containing histidine tags and amine-coupling of NeutrAvidin to capture biotinylated ligands.
What tag is recommended for proteins to use in SPR?
For small molecule binding studies, we recommend the target protein to have Avi-Tag. Biotinylated proteins allow rapid immobilization using Neutravidin sensors and are ideal for small molecule binding studies.
How much ligand is used in SPR?
For immobilization you only need ~25-50ug of ligand in a suitable buffer. If using amine coupling the buffer should not contain free amines (e.g. TRIS buffer is not suitable). The stock ligand concentration should be sufficiently high (>0.5mg/ml) because the ligand will be diluted in immobilization buffer.
How much analyte is used in SPR?
It depends on the binding kinetics between ligand and analyte. For kinetic studies, a range of concentrations between 0.01-100 times the KD is injected.
What is the make/model of LifeSensors' instrument?
We use a Reichert SPR4 instrument which supports a four-channel flow cell. This allows up to 3 experimental channels with one reference channel. The 3 experimental channels are isolated from each other, allowing for up to three different ligands to be screened. Data is processed using TraceDrawer.
How long does it take to immobilize a ligand on the chip?
Depending on the immobilization protocol, about 45-90 minutes total is needed to prepare a chip. When the immobilization conditions are known, a simple amine coupling can be done within 30 minutes.
How much ligand immobilized onto the sensor chips?
The amount of ligand depends on the application. Protein-protein interactions typically require less surface density of ligand (ca. 1000-4000 RU). To measure small molecule (MW >300 Da) binding to proteins, one typically wants a saturated ligand surface; ~8000-10,000 RU of captured target protein ligand should be sufficient.
Which coupling buffer is used in SPR?
For the standard amine-coupling, 10mM sodium acetate (pH 4.0-5.5) buffer is used. Ligand storage buffer should not contain free amines (e.g. TRIS).
What regeneration buffers are used in SPR?
Regeneration buffers often need to be screened depending on the type of SPR experiments and interactions measured. Some typical regeneration solutions include 0.5-1M NaCl (ionic), 50 mM Glycine (pH 3.0 or 9.5), ethylene glycol (hydrophobic), or some combination thereof.
Can I get binding affinity KD for my small molecule compound and target protein of interest using SPR?
Yes. SPR can reliably determine the binding affinity KD for small molecules and target proteins. Typically, the target protein of interest is captured onto the sensor chip and a series of compound doses are injected to generate dose-response binding curves.
