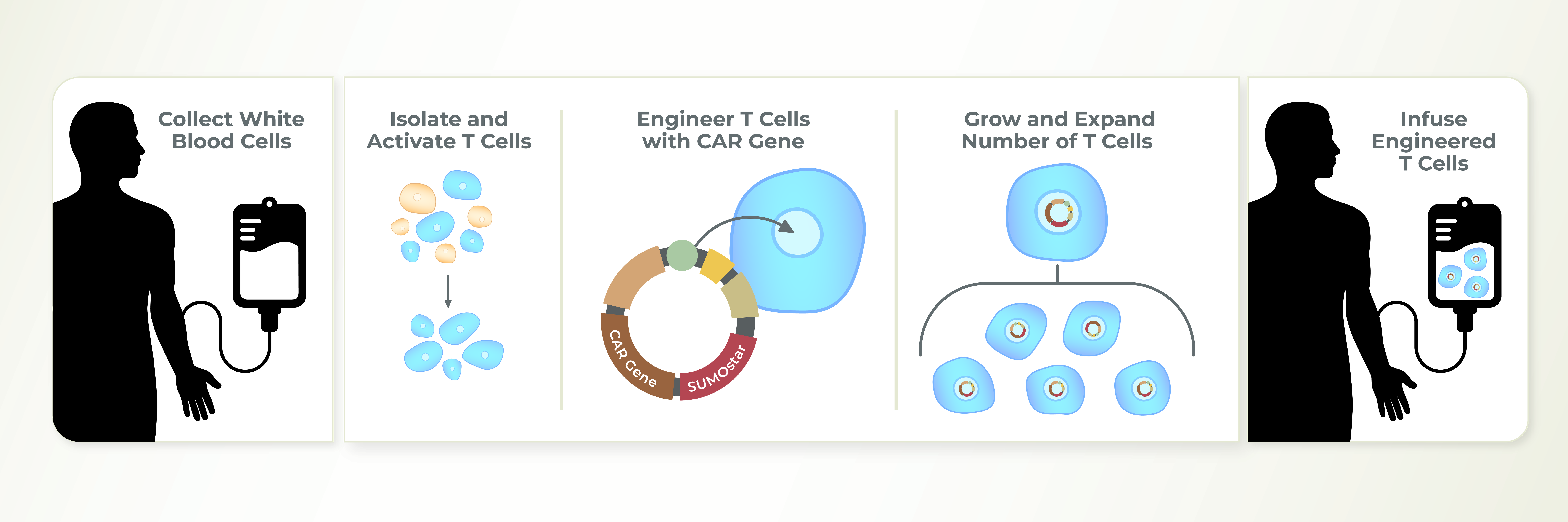
Many new therapies are now being delivered as genes, mRNA, or AAV-mediated vehicles. Poor expression in prokaryotic and eukaryotic hosts is the primary cause of a lack of efficacy of these therapies. Clinical-scale production of functional biotherapeutics such as proteins used in CAR-T therapy and vaccine candidates routinely faces challenges of expression quantity, misfolding, incorrect localization, degradation in the host cell, and HIGH costs. However, clinical-scale production remains critical for successful therapeutic outcomes.
To overcome these challenges, we developed the SUMOstar™ tag, a modified version of the SUMO fusion platform ideal for mammalian expression due to its resistance to endogenous SUMO proteases found in all eukaryotic systems. The SUMOstar™ tag remains tethered to the protein throughout the purification process. Precise C-terminal removal of the tag simply requires end user-induced cleavage by engineered SUMOstar™ proteases. The native N-terminal residues of the protein are completely preserved following cleavage, which is particularly important for researchers seeking biologically active cytokine-based therapeutic strategies.