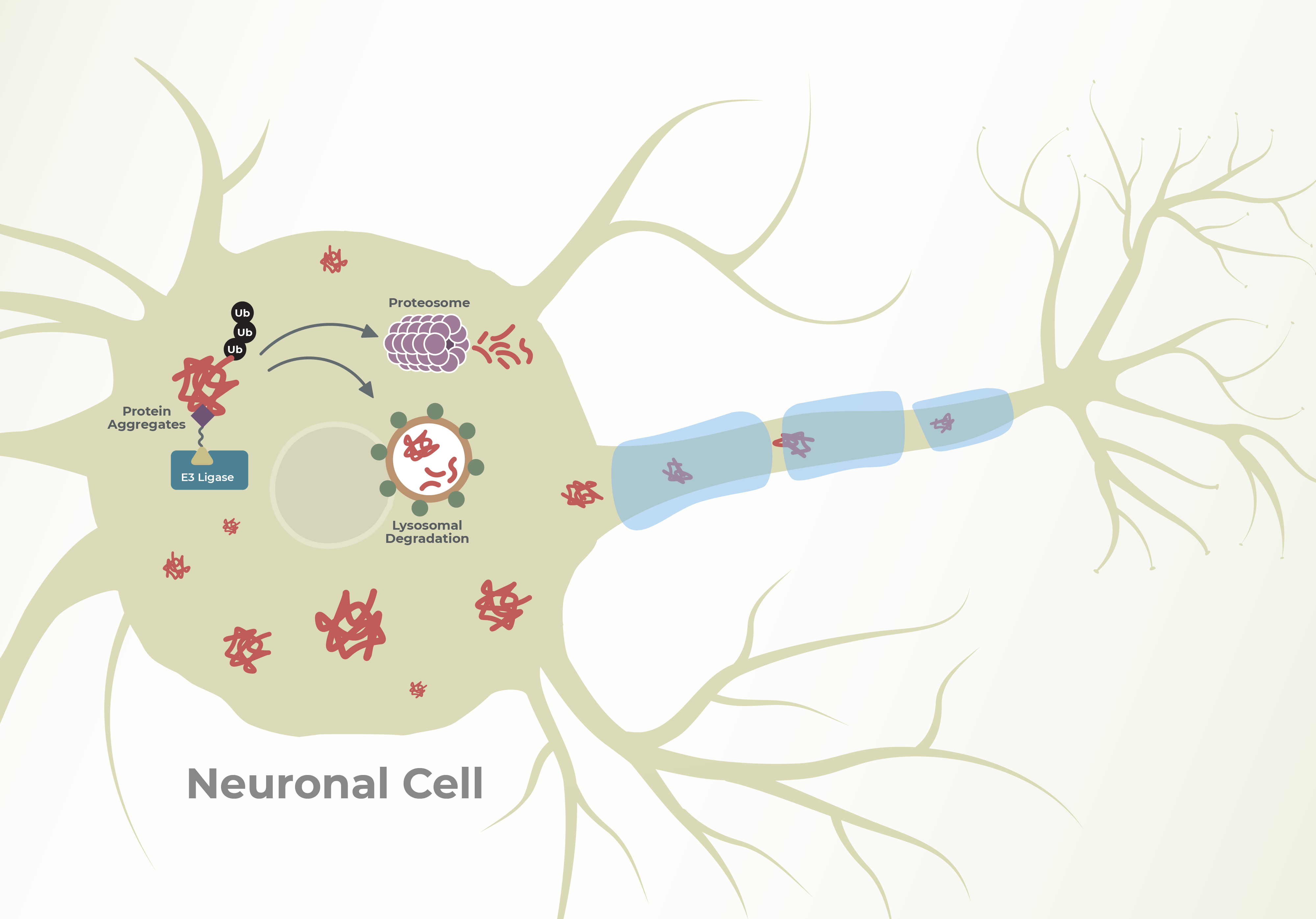
A wide range of neurodegenerative diseases affect the central nervous system (CNS) and lead to impaired sensory, motor, and cognitive processes. This impairment can lead to permanent damage to neuronal health and eventual neuronal death. The complex disease pathophysiology and poorly studied underlying mechanisms have led to an increase in therapeutic efforts, which have historically been proven to be difficult. Proteolytic Targeted Chimera (PROTAC®) protein degraders open up new and exciting opportunities for degrading disease-causing mutants and protein aggregates, starting with tauopathies and synucleinopathies. Some hallmarks of the new generation of PROTACs are improved pharmacology to cross the blood-brain barrier (BBB), increased catalytic activity to break down aggregates to prevent diffusion to healthy neurons, and the ability to adapt to new delivery mechanisms. There is ample evidence that large protein aggregates are proteasome-resistant, so PROTACs that selectively drive degradation through the autophagosomal mechanism can significantly improve therapeutic outcomes. It is of great significance to study novel E3 ligases compared to traditional E3 ligases to generate polyubiquitin chains that can promote both proteasomal and lysosomal degradation. Lifesensors has developed custom high-throughput assays to study PROTAC-mediated ubiquitination and degradation of protein aggregates to provide mechanistic insights that help chemists design reliable SARs.
Tauopathies are neurodegenerative disorders marked by the buildup of abnormal tau proteins, ultimately resulting in neuronal demise. A recent innovative study sheds light on the pathogenesis of tauopathies and offers potential therapeutic strategies targeting tau protein degradation.¹ Explore groundbreaking research on tauopathies conducted by Silva, M. C., et al., as presented in their paper “Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models.”
Another groundbreaking study by Wang W, et al. presenting a PROTAC degrader aimed at selectively degrading tau proteins implicated in Alzheimer’s disease (AD) and related tauopathies. Their small-molecule PROTAC, C004019, effectively promotes tau clearance both in vitro and in vivo. This study demonstrates significant reductions in tau levels in the brains of wild-type, hTau-transgenic, and 3xTg-AD mice following administration of C004019. This reduction in tau correlates with improvements in synaptic and cognitive functions, highlighting the therapeutic promise of C004019 PROTAC for AD and tauopathies.² This study signals a new era in drug development targeting tau pathology, offering hope for effective treatments in neurodegenerative diseases.