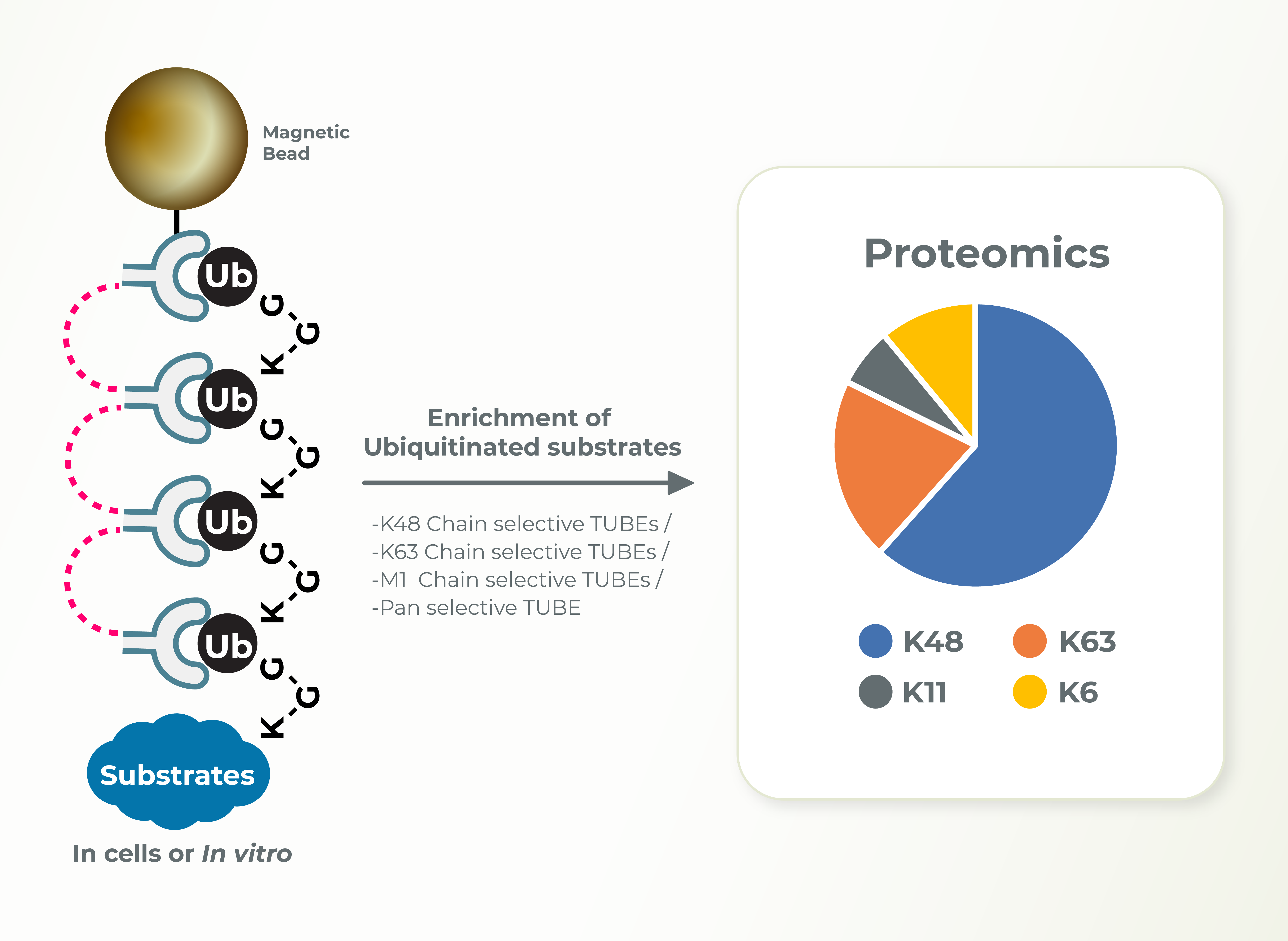
The Ubiquitin Proteasome System (UPS) controls the principal functions of almost all the cellular proteins of human cells and failures in this system often contribute directly or indirectly to the pathogenesis of many diseases, including cancer, inflammation, and neurodegeneration. There are ~700 E3 ubiquitin ligases encoded in human genome and the functions of most of these E3s remain unknown. E3 ligases build diverse ubiquitin chain linkages on substrates that can determine the fate of the substrate. Whereas K48-linked ubiquitin chains promote proteasomal degradation of substrates, K63-linked ubiquitin chains are associated with signaling and localization. Identification of the type of ubiquitin linkage can provide insights into the cellular functions of E3s and substrates. Lifesensors can perform these studies using linkage specific TUBEs very efficiently and in a timely manner for the clients.
Service Highlights
- Cell-based assays to identify endogenous substrate ubiquitination
- Identify the diversity of ubiquitin chain linkages
- Study the effect of PROTACs or small molecules chain diversity
Related Products
TUBE1
Coated polymeric high-capacity magnetic beads to allow superior enrichment of polyubiquitinated proteins along with minimizing non-specific binding to proteins in tissue and cellular lysates.
Agarose-TUBE1
TUBEs display up to a 1000-fold increase in affinity for polyubiquitin moieties over the single ubiquitin binding associated domain (UBA). In addition, TUBEs protect polyubiquitinated proteins from deubiquitination and proteasomal degradation, allowing for detection at relatively low abundance.
VU1
Monoclonal antibodies that recognize polyubiquitylated proteins and free ubiquitin. VU-1 has been shown to recognize all ubiquitin linkages (mono, K6, K11, K27, K29, K33, K48, K63, and linear) by Western blotting. VU-1 is an excellent antibody for immunostaining applications generating robust ubiquitin detection and low background with an ability to detect subcellular ubiquitin localization.