UBIQUITIN: HOPE FOR THE FUTURE OF ALZHEIMER’S RESEARCH
by: Mark Tingey and Peter Foote
Uncle Walt stared at us, not recognizing us or remembering our names. His eyes, which were once bright and filled with curiosity, were now fearful and withdrawn. He appeared to crumple in upon himself, as if the world he no longer recognized was crushing him. Walt was in the late stages of Alzheimer’s Disease (AD). I had thought my education on the topic would inure me to the impacts of the disease; rarely have I been so wrong. The realities of Alzheimer’s Disease are much harsher than any academic journal could ever portray.
Prior to his disease, Walt had been a brilliant researcher at Washington University. In a decorated career, the thing he was most proud of was assisting in the development of In Vitro Fertilization (IVF). This technique has since blessed the lives of tens of thousands of people worldwide. However, the man who now huddled in his chair had no memory or recollection of his greatest achievement. He had lost the ability to recognize his family, his friends, and he no longer was able to recognize himself. He was slowly being robbed of his entire being by Alzheimer’s Disease.
The story of Uncle Walt is unfortunately very common. All throughout the world, people are resigned to watch family and friends slowly fade away as the AD progresses. This disease often instills family members with a sense of hopelessness and a feeling of futility. I felt this feeling when I watched Uncle Walt slowly disappear and eventually leave us forever. This is why I am writing this article. The future of AD research is bright. There is hope for the future, and that hope is due to the recognition that Alzheimer’s is a fundamental breakdown of the ubiquitin pathway.
The Current Paradigm in Alzheimer’s Disease diagnosis
To explain how the ubiquitin pathway holds the key to early AD diagnosis and potential treatment, we must first discuss how things are currently done. For decades researchers have operated under two paradigms which have hampered advances in the field. First, the diagnosis of AD is very difficult. Clinicians currently must wait for the disease to advance sufficiently before being able to provide a confident diagnosis. This has left clinicians to rely upon an archaic system. The current system essentially breaks the progression of symptoms into stages ranging from no impairment to severe impairment. Confirmation of the diagnosis is only possible through a combination of brain scans and cognitive tests. This diagnostic scheme is too slow to allow patients to receive proactive or interventional treatment, as insurance providers will not pay for treatments until a diagnosis is confirmed.
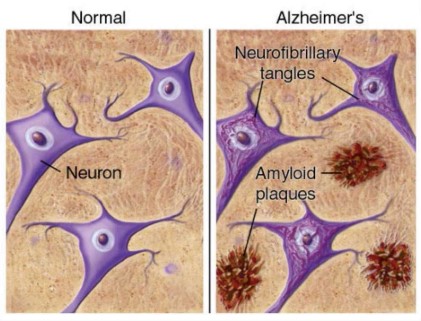
Second, researchers and clinicians have been focusing on the build-up of detrimental protein which leads to AD symptoms. Neuronal cells are often considered to be the information highway of the body. In neurodegenerative diseases, such as Alzheimer’s Disease, neuronal cells produce too much of certain proteins. If these neuronal cells are a highway, then the excess proteins are causing a traffic jam. The two major culprits are β-amyloid and Tau. β-amyloid is secreted by the neuronal cells and congregates in plaques. These plaques block the transmission of signals between neuronal cells and lead to impairment. Tau proteins exist inside the cell, and normally help facilitate the cell’s transport system. When too many Tau proteins are produced, they form tangles within the cell. This interferes with the cells vital functions, and often leads to the death of that cell. Researchers are so focused on how these proteins negatively impact people with AD, that they aren’t looking at why so many proteins are being produced in the first place.
The Hunt for an Accurate Biomarker
Every function in your body, good and bad, is carried out by proteins. In most diseases, the easiest way to determine if a patient has this disease is to identify the malfunctioning protein and then test the patient for this protein. This approach is what is commonly referred to as the Biomarker approach. The protein would be considered a unique biomarker for that disease. For the test to be successful, the biomarker will always be found in people with the disease and will never be found in people who do not have the disease.
For decades, researchers have tried to identify a biomarker that would allow for AD diagnosis. The problem researchers have found is that the biomarkers for AD are not simple. The assumption was that AD progression was due to the overproduction of a protein called β-amyloid. It was thought for a long time that this protein would be a perfect biomarker for AD. Unfortunately, it has been clinically shown that some people form β-amyloid plaques and have no symptoms of AD. Similarly, there are people with AD who have no β-amyloid plaques.
Tau was also evaluated as a possible biomarker but failed in the same way that β-amyloid failed. Many other proteins have been evaluated but could not successfully meet the criteria of only being present in diseased individuals, and never present in healthy individuals. The high rate of false positives has rendered many of the known proteins ineffective and not suitable as an accurate biomarker. The search for an accurate biomarker is ongoing and difficult. In fact, many researchers are beginning to believe that a biomarker for AD will not be a single protein, but a group of proteins.
Ubiquitin is the key to Alzheimer’s Research
Alzheimer’s Disease, like many such diseases, is a fundamental breakdown of the ubiquitin pathway. Ubiquitin is a protein which will interact with every other protein in the cell at one point or another. One of the most important tasks of ubiquitin is to tag proteins that are being produced too often, or are malfunctioning, for degradation. For instance, in a healthy cell producing too much Tau protein, the ubiquitin proteasome system (UPS) will tag the excess proteins and they will be destroyed. When the UPS is functioning properly, aggregation of proteins such as β-amyloid and Tau would be degraded prior to being able to negatively impact the body as a whole.
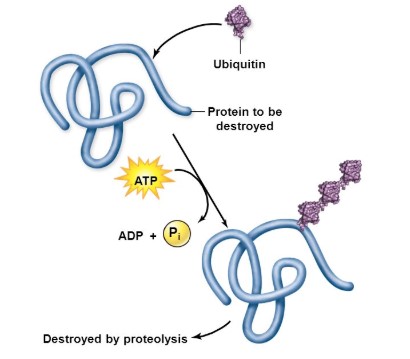
We at LifeSensors, as well as many researchers around the globe, have come to recognize the importance of understanding the ubiquitin proteasome system in relation to AD. The root cause of AD is too many proteins causing negative effects in the brain. This represents a fundamental breakdown in the ubiquitin proteasome pathway. These excess proteins are not being tagged for degradation at a quick enough pace. Focusing on the ubiquitin proteasome system will enable researchers to identify the unique changes which are the root cause of this breakdown. In addition to helping researchers develop medications, understanding this relationship is the key to finding an effective biomarker.
Innovative Research Technologies
The search for effective diagnostic and treatment tools has been greatly helped as technology advances. As the role of ubiquitin in AD becomes to be more firmly substantiated, innovative research technologies are required which will help researchers unravel the UPS-AD relationship. In our effort to assist researchers, LifeSensors has worked to develop Tandem Ubiquitin Binding Entities, or TUBEs, for short. TUBEs are protein tools based on nature’s own ubiquitin detectors; we have simply repurposed these detectors and grouped them together with some slight modifications for our own use. TUBEs allow us to isolate and detect ubiquitin and any proteins that it has tagged. This means we can use TUBEs to understand which proteins are tagged by ubiquitin in samples taken from AD patients. One or more of these tagged proteins may turn out to be the elusive biomarker that lets researchers distinguish between healthy and AD-afflicted patients, even before the disease starts to show itself.

Discovering biomarkers could be a monumental task. Identifying a few proteins out of thousands will be made easier with TUBEs, but LifeSensors scientists will still need to collaborate with other leading researchers and make use of their specific expertise. Mass spectrometry-based proteomics is one of many techniques that our collaborators use to help us find individual biomarkers in complex mixtures like human blood or spinal fluid. Once we discover the needles in these proverbial haystacks, we will use our expertise in ubiquitin to recommend drug targets that will allow researchers to treat, prevent, and cure AD.
Hope for the future
Alzheimer’s Disease is a mammoth problem which looms large in the hearts and minds of people the world over. In recent years, researchers have begun to target AD. Unfortunately, they have found a series of seemingly insurmountable hurdles. The first hurdle is finding a reliable biomarker which will allow for accurate and early diagnosis of Alzheimer’s Disease. The ubiquitin proteasome system is the key to finding this biomarker. The unique breakdown in ubiquitin processing which enables AD to progress is also the key to identifying AD when it is in its infancy.
LifeSensors is committed to developing methods to identify this biomarker. We firmly believe that the power of our research technology, our passion for improving the world, and our visionary ideals will lead to the identification of an AD biomarker. This discovery will enable clinicians to proactively begin interventional therapies which will dramatically improve the quality of life for those afflicted with this terrible disease.
For more information on AD, please consult the Alzheimer’s Association or the National Health Institute. To collaborate with LifeSensors in our efforts to identify AD biomarkers please fill out a form here.
