By: Isabelle Wentzel
Devastating Origins
In the late 1950s and early 1960s, pregnant women who experienced nausea and morning sickness were prescribed thalidomide, a sedative drug meant to treat these symptoms. In early 1960, it quickly became evident that there was a link between the thousands of infants born with severe birth defects and malformations and the in-utero exposure to thalidomide. The drug was eventually pulled from the markets of most countries in the early 1960s.
A Glimpse into the Future
It wasn’t until 60 years later that scientists discovered thalidomide’s mechanism with regard to birth defects. Researchers found that the thalidomide drug mechanism acted by aiding degradation of a range of transcription factors, including SALL4, which is involved in limb development and fetal growth. It was later discovered that SALL4 degradation may also be linked to antiangiogenic activity, which prevents or slows the growth of blood vessel formation in tumors, potentially inhibiting tumor growth. Thalidomide was later used to treat multiple myeloma. At the time, researchers didn’t realize thalidomide was a molecular glue. How its history would later strike a match in the emerging field of targeted protein degradation was yet to be seen.
The Rise of PROTACs and Molecular Glues
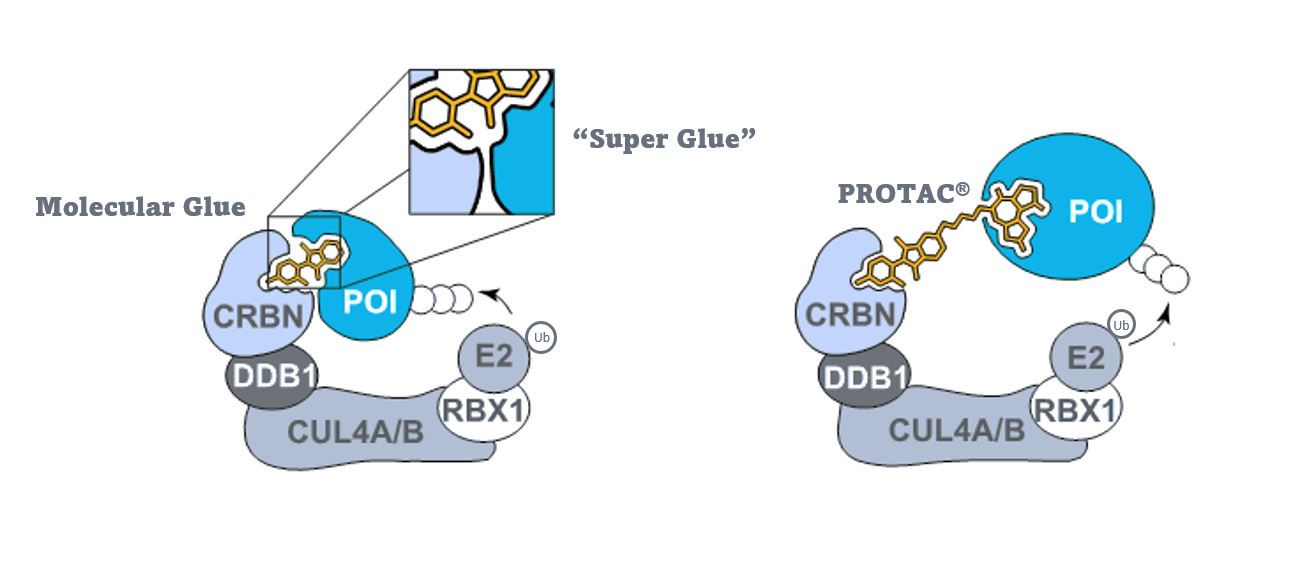
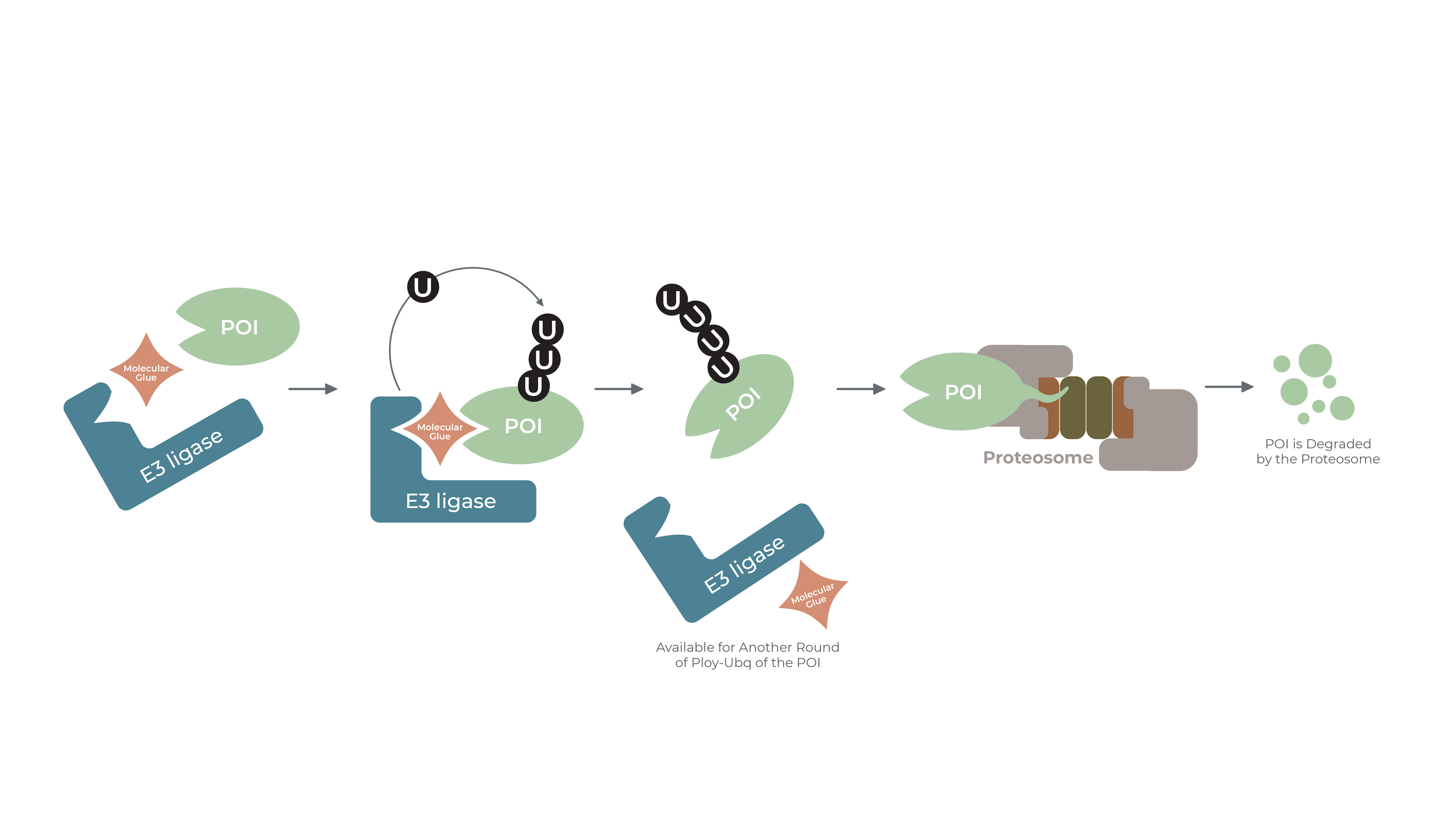
Targeted protein degradation (TPD) is a therapeutic paradigm that hijacks the natural mechanism of the ubiquitin proteasome system (UPS) to degrade a target protein. Molecular glues are small chemical entities that destabilize a target protein of interest by binding to an E3-ubiquitin ligase, altering its shape, and then bind to the target protein which begins the degradation cascade. The E3-ligase tags the target protein with a chain of ubiquitin molecules, which signals the target protein for degradation by the proteasome. The molecular glue mechanism of action is quite alluring, as it enables the targeting of proteins otherwise considered “undruggable.” This type of research is now surging through the biotech community, as scientists are attempting to discover and develop molecular glues to target and degrade a myriad of disease-causing proteins that until now have been neglected due to lack of known therapeutic entities.
The molecular glue approach is similar to the concept of a PROTAC (Proteolysis-Targeting Chimeric Molecule): which is a hetero-bifunctional small molecule containing two functional ligands – an E3-ligase and a target protein – connected via a linker. Like molecular glues, in theory, bringing these two entities together leads to the polyubiquitylation and proteasomal degradation of the target protein. Unlike classic small molecule inhibitor drugs, PROTACs can be used again and again, allowing for lower dosage and efficient treatment.
Small But Mighty
So, what makes these molecules different and why are molecular glues now reigning over PROTACs? The major difference between PROTACs and molecular glues is that PROTACs are bifunctional molecules, connected by a linker, while molecular glues are smaller and made up of a single molecule. While both modalities are effective, molecular glues inherently have an advantage over PROTACs, as they resemble traditional small molecule drug design principles. Being a smaller molecule, a molecular glue would intrinsically pose qualities such as better solubility and membrane permeability, based on basic druggability principles.
Challenging Targets Meet Their Match
With incredible excitement surrounding the ever-growing field of targeted protein degradation, there is an unmet need for truly functional assays that are designed to study the effects of molecular glues on ubiquitination of target proteins and their degradation. While the traditional methods of western blotting and reporter gene assays are adequate to study target protein degradation, they are immensely low throughput, time consuming, and prone to artifacts. LifeSensors has developed high-throughput methods that are designed to directly monitor molecular and PROTAC mediated ubiquitylation and degradation of target proteins.
LifeSensors is well-equipped to help scientists with their challenges related to the field of targeted protein degradation. With a strong expertise in medicinal chemistry, biochemistry, and cell-biology specializing in ubiquitin-based drug discovery for the past 15 years, we are proud to offer valuable methods and a variety of services to accelerate your discovery of novel molecules facilitating targeted protein degradation.
If you are interested in exploring the capabilities that LifeSensors can bring to your research, get in touch with our team here.