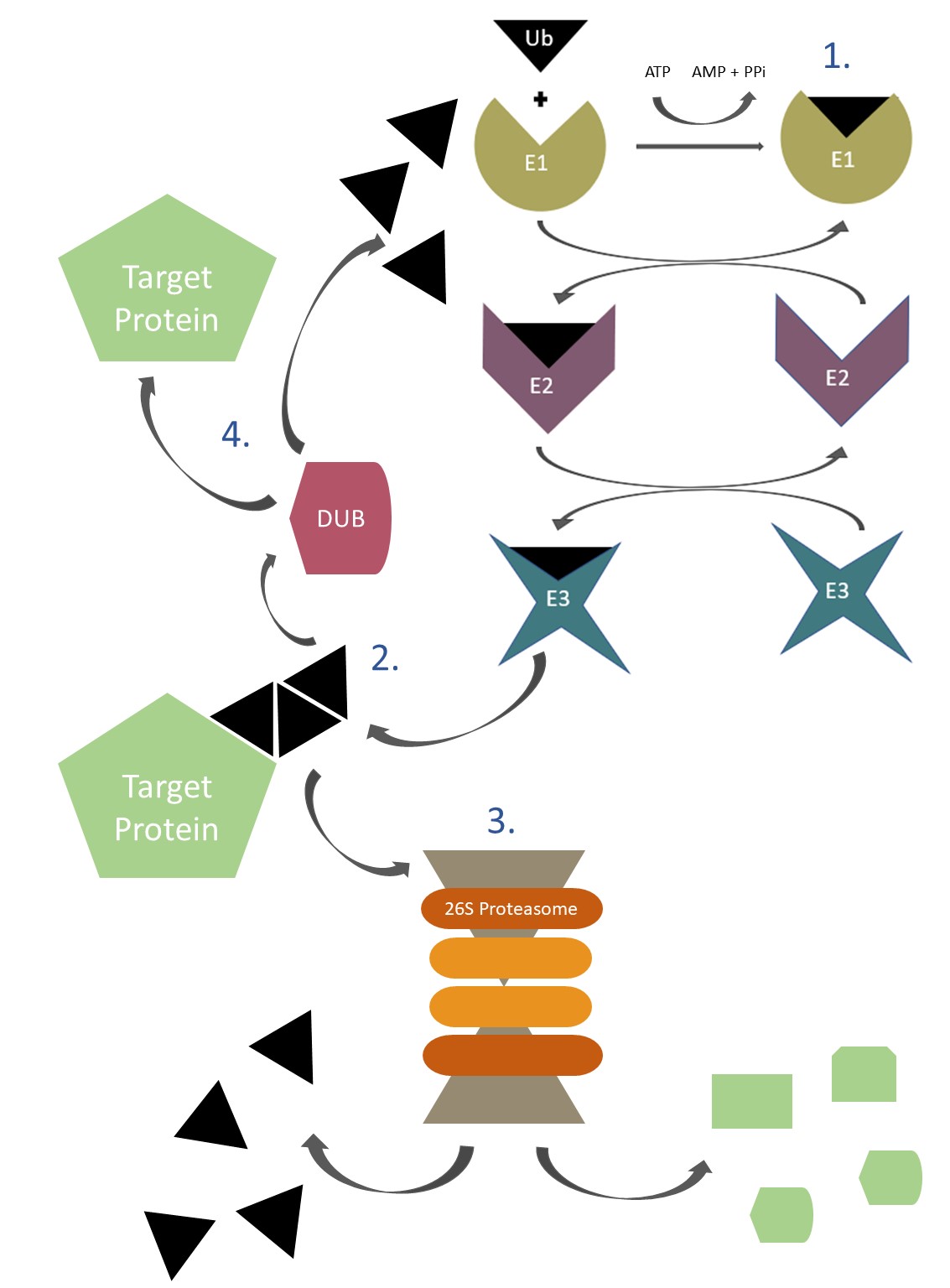
The ubiquitination reaction is a sequential process mediated by three enzymes (E1, E2, and E3).
- First, ubiquitin is activated in an ATP-dependent reaction catalyzed by the E1 activating enzyme. The activated ubiquitin is then transiently carried by the E2 conjugating enzyme, which, along with the E3 ubiquitin ligase, transfers the ubiquitin to its specific substrate.
- It is accomplished by formation of an isopeptide bond between a lysine residue in the target protein and the C-terminal glycine of the ubiquitin. The E3 ubiquitin ligases confer substrate specificity for ubiquitin ligation. Mammalian cells typically contain a few E1s, 30–40 E2s and several hundred different E3 ubiquitin ligases. The complex interplay between the E1, E2 and E3 ubiquitin ligases allows for an enormous number of substrates to be modified and thereby contributes toward the specificity and diversity of the ubiquitination process.
- In most of the cases, ubiquitin-tagged proteins are directed towards the proteasome for degradation.
- The process of ubiquitination is reversible, and the reverse process is called deubiquitination which is accomplished by deubiquitinating enzymes (also known as DUBs). DUBs play an important role in regulation of the UPS function including proofreading ubiquitin-protein conjugates and removing ubiquitin from conjugated proteins.