Pozhidaeva A, Bezsonova I, USP7: structure, substrate specificity, and inhibition, DNA Repair (2019), https://doi.org/10.1016/j.dnarep.2019.02.005
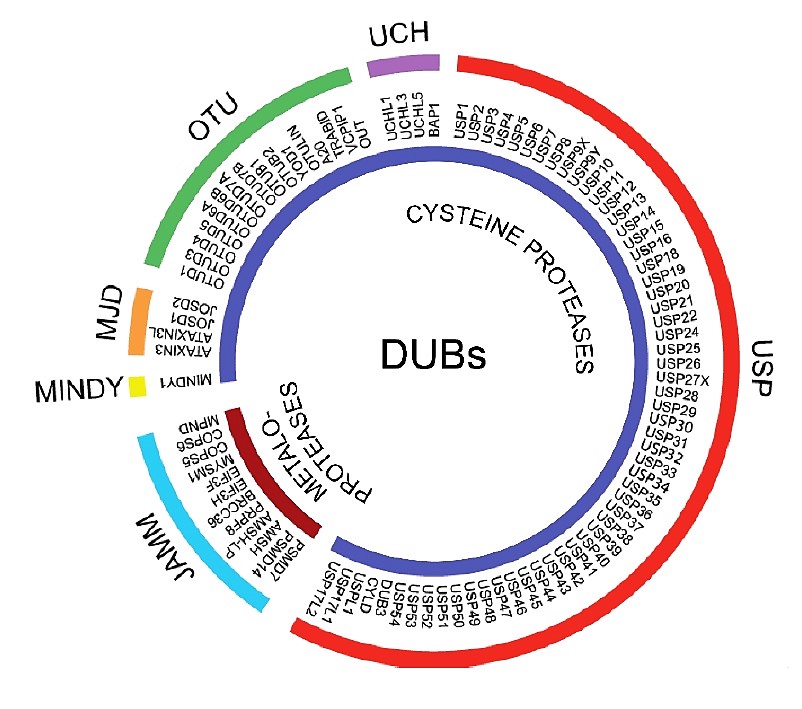
Deubiquitinases or deubiquitinating enzymes (DUBs) can reverse the effect of E3 ligases by removing ubiquitin from target proteins. They are also involved in ubiquitin maturation, recycling and editing. The human genome encodes ∼100 DUBs. Based on sequence and domain conservation, DUBs can also be divided into six subfamilies: ubiquitin-specific proteases (USPs), ovarian-tumor proteases (OTUs), Machado–Joseph disease protein domain proteases (MJDs), ubiquitin carboxyterminal hydrolases (UCHs), monocyte chemotactic protein-induced protein (MCPIP) and JAMM/MPN domain-associated metallopeptidases (JAMMs). Their substrates and the regulatory mechanisms, biological functions, and particularly the roles of they play in a variety of clinical diseases, has become increasingly appreciated. Unlike most E3 ligases, DUBs have an inherent catalytic activity that more easily targeted by small molecules.
