Surface Plasmon Resonance
Advanced SPR technology for precise ligand-protein binding analysis and drug discovery applications
Need Help?

- What is Surface Plasmon Resonance?


Generating experimental data for ligand binding to target protein of interest as well as determination of ligand binding affinity (Kd) is essential for drug discovery. SPR is a label-free optical technique that measures interactions in real time. A target of interest (ligand) is immobilized on a thin gold-coated glass chip while a second molecule (analyte) is flowed over the chip. Changes in the refractive index at the gold surface as the analyte binds to and dissociates from the ligand are monitored to generate sensograms, from which kon, koff, and KD can be calculated.
- Why SPR?
Drug Discovery Applications
Generating experimental data for ligand binding to target protein of interest as well as determination of ligand binding affinity (Kd) is essential for drug discovery. SPR is a label-free optical technique that measures interactions in real time. A target of interest (ligand) is immobilized on a thin gold-coated glass chip while a second molecule (analyte) is flowed over the chip. Changes in the refractive index at the gold surface as the analyte binds to and dissociates from the ligand are monitored to generate sensograms, from which kon, koff, and KD can be calculated.
Expert Team & Advanced Equipment
- 13,200
- 0.02 RU
Drug Discovery Applications
We use the Bruker Sierra SPR-32 Pro high-throughput SPR analyzer that can enable 13,200 interactions studies (4400 samples) per day. Their breakthrough SPR+ detector allows for superior sensitivity with signal-to-noise ratio of 0.02 RU derived as a function of imaging SPR (SPRi) and high-speed optical scanning. It is crucial to have best signal-to-noise ratio when screening for identifying and characterizing novel small molecule ligands and PROTAC studies to study binary interactions.
- Applications & Capabilities
Protein-Protein Interactions
Comprehensive analysis of protein binding dynamics
Small Molecule Binding
Precise characterization of ligand interactions
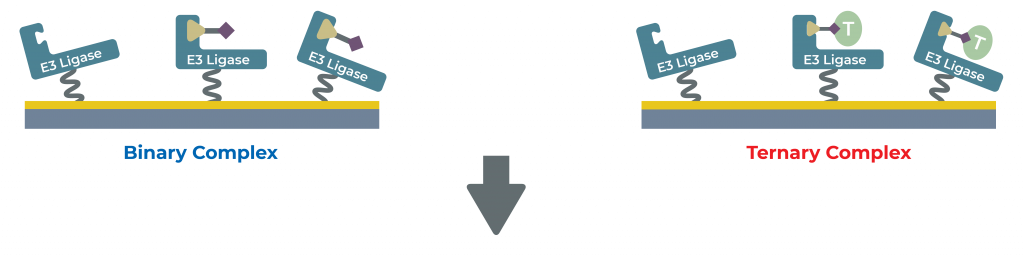
PROTAC Studies
Binary and ternary complex formation analysis
Kinetic Analysis
Real-time kon, koff, and KD determination
- Service Highlights
- Tailored solutions to study protein-protein, protein-small molecules/PROTAC interactions
- Characterization of small molecule and PROTACs binding to E3 and target proteins
- Characterization of E3 and DUB ligands and demonstrate selectivity
- High-throughput screening with kinetics and affinity reporting (kon, koff, and KD)
