Neurodegenerative diseases are often characterized by excessive or dysfunctional proteins and protein aggregates, which induce specific pathological conditions. The ubiquitin proteasome system and autophagy play key roles in the regulation of protein homeostasis in neurons. Accordingly, understanding enzyme dysfunction in the ubiquitin proteasome system and autophagy is critical to understanding and ultimately treating a variety of neurodegenerative disorders.
Neurons are somewhat unique in that they do not divide, proliferate, or create new neuronal cells to replace dysfunctional ones. Because of this lack of neuronal replenishment, maintaining proteostasis is critical for neuronal cell health and function. When proteins are over-produced, misfolded, or aggregated in a detrimental manner, the Ubiquitin Proteasome System (UPS) provides a mechanism for clearing them.
While the UPS is a robust and incredibly important system for maintaining proteostasis, it is not the only pathway by which proteostasis is maintained. To clear organelles or protein aggregates, the cell utilizes autophagic protein degradation (APD). Recent evidence indicates considerable crosstalk between these two systems, and UPS enzymes play a key role in both systems of degradation.
The overproduction and aggregation of proteins in the neuron is well documented, particularly those related to the pathogenesis of Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). These neurodegenerative diseases are widespread, high-profile, and heavily studied. In Alzheimer’s Disease, Tau and Amyloid proteins have been found to aggregate, indicating a fundamental failure of the UPS and APD. In addition to the inability of these systems to maintain proteostasis, ubiquitin is further implicated in Alzheimer’s Disease by reports of differential ubiquitylation in AD brains.
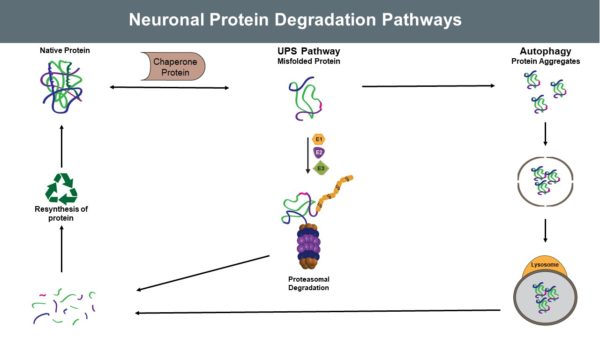
Assaying the UPS and APD is critical to understanding the etiology of these diseases. However, regardless of the specific disease, it is important to probe brains for differential ubiquitylation. Many researchers hypothesize that differential ubiquitylation functions in tandem with specific proteins as an effective diagnostic biomarker. Antibodies, however, are often unsuitable for detecting ubiquitylated proteins, particularly because linkage-specific antibodies often exhibit poor selectivity. We have found that utilization of TUBE technology allows for accurate assay of brain specific ubiquitylation.
Autophagy is critical for the removal of protein aggregates and damaged organelles within the brain. In particular, mitochondrial depolarization is associated with the development of PD. The E3 ubiquitin ligase Parkin is critical to the initiation of mitochondrial autophagy (mitophagy), which removes damaged mitochondria. The kinase PINK1 and the DUB USP30 are also critical players in this pathway. While many types of ubiquitin chain are relevant to autophagy and mitophagy, K6-linked ubiquitin chains and phospho-ubiquitin are among the most critical. In addition to tools specific to Parkin-mediated mitophagy, LifeSensors also provides a variety of more general autophagy-related products.
Understanding an E3 ligase is an essential step for choosing a target for screening in the case of a molecular
With ubiquitin’s core functions in plants and animals being so similar it has long been speculated that insights into the
The functional consequences of protein polyubiquitination are determined by the type of ubiquitin chain assembled on the substrate. Among the
While direct K-RAS inhibitors represent a major therapeutic advance, targeting this oncoprotein remains a significant challenge due to limited druggable
Pan-selective Tandem Ubiquitin-Binding Entities (TUBEs) are engineered, high-affinity reagents composed of multiple ubiquitin-associated (UBA) domains that bind polyubiquitin chains with
LifeSensors is pleased to bring attention to some of our academic colleagues who have used our products in the past
Understanding an E3 ligase is an essential step for choosing a target for screening in the case of a molecular
With ubiquitin’s core functions in plants and animals being so similar it has long been speculated that insights into the
The functional consequences of protein polyubiquitination are determined by the type of ubiquitin chain assembled on the substrate. Among the
While direct K-RAS inhibitors represent a major therapeutic advance, targeting this oncoprotein remains a significant challenge due to limited druggable
Pan-selective Tandem Ubiquitin-Binding Entities (TUBEs) are engineered, high-affinity reagents composed of multiple ubiquitin-associated (UBA) domains that bind polyubiquitin chains with
LifeSensors is pleased to bring attention to some of our academic colleagues who have used our products in the past