FDA Provisional Approval
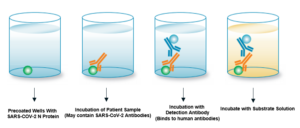
LifeSensors is proud to announce its provisional FDA approval for our COVID-19 IgG ELISA Detection Kit to address the critical need for COVID-19 diagnostics through serological testing. Establishing the infection history of SARS-CoV-2 individuals will assist identifying those likely to transmit the disease, effectively halting the spread of the virus. The COVID-19 IgG ELISA Detection Kit is a quick and robust technology that is compatible with large population testing. LifeSensors is dedicated to the detection of the long-term serological antibody sub-type, IgG and our affordable comes with many advantages.
Advantages of Our IgG ELISA Detection Kit
- Detects active or past infection (patient exposure status)
- Rapid results (< 90 minutes)
- Increased levels of sensitivity and specificity (detects antibodies to virus at low femtomole concentrations)
- Suited to any size laboratory or clinic (compatible with simplest plate readers)
Contact Us
Stayed tuned for additional serological detection kits from LifeSensors!
If you have any questions or interest in our kit for clinical or lab use, please reach out to bd@davidk162.sg-host.com/lifesensors or call (610) 644-8845 ext 339
